Resorbable vs. Non-Resorbable Bone Grafts
Written By: Dr. Sneha Dhanke
How CERASORB® M excels in both Dental and Orthopedic applications
Bone grafting plays a critical role in both dental and orthopedic procedures, providing structural support and facilitating natural bone regeneration. Among the various types of bone grafts available, resorbable grafts, such as CERASORB® M, offer significant advantages over traditional Xenografts and Allografts.
In this article, we explore how CERASORB® M, a β-TCP-based biosynthetic bone regeneration material, outperforms other grafting materials in both oral surgery and orthopedic applications.
Resorbable vs. Non-Resorbable Bone Grafts: What’s the Difference?
Non-Resorbable Grafts: Xenografts & Allografts
- Xenografts (bovine or porcine-derived bone) and Allografts (cadaveric human bone) serve as scaffolds for new bone formation but are not completely resorbed over time i, ii, iii, iv
- They require the host’s bone to grow around and incorporate the graft material, which may remain in the body indefinitely
- These materials carry potential risks such as disease transmission, immune reactions, or incomplete integration v, vi, vii, viii
Resorbable Grafts: The Power of β-TCP-Based CERASORB® M
- CERASORB® M is a fully biosynthetic β-tricalcium phosphate (β-TCP) graft that completely resorbs and gets replaced by natural bone over time ix, x, xi
- It is free from biological contaminants, eliminating concerns of disease transmission or immune reactions xii
- Its porosity (macro, meso, and micropores) allows for vascularization, cell migration, and bone remodeling, ensuring a seamless transition to healthy native bone xiii
How CERASORB® M integrates into native bone over time
One of the key benefits of CERASORB® M is its controlled resorption and replacement by natural bone. The process unfolds in three key stages:
1. Initial Implantation:
- The graft serves as an osteoconductive scaffold, guiding bone cells into its porous structure
- Its interconnected macroporous, mesoporous, and microporous structure promotes blood vessel infiltration, a critical step for bone formation
2. Bone Regeneration Phase:
- Osteoclasts gradually break down the β-TCP granules, while osteoblasts form new bone in their place
- This process mimics natural bone turnover, ensuring gradual and predictable integration
3. Complete Resorption & Bone Remodeling:
- Over several months, CERASORB® M fully resorbs, leaving behind only strong, vital bone
- Unlike Xenografts or Allografts, no foreign material remains, reducing long-term complications
Performance Comparison: Oral Surgery vs. Orthopedic Applications
| Feature | Dental Applications | Orthopedic Applications |
| Bone Regeneration Time | 4 – 6 months, faster due to smaller defects | 6 – 12 months, longer due to load-bearing sites |
| Graft Stability | High, as it maintains space for bone growth | High, ensuring mechanical support in bone defects |
| Resorption Rate | Complete resorption within 4 – 6 months | Controlled resorption based on bone turnover rate |
| Clinical Benefits | Ideal for socket preservation, sinus lift, and ridge augmentation | Used in trauma and joint reconstruction |
CERASORB® M: An Effective Graft Extender for Bone Regeneration
CERASORB® M enhances autografts and allografts, maximizing graft volume while promoting efficient bone regeneration through its osteoconductive, multiporous structure.
Why Use CERASORB® M as a Graft Extender?
Key Benefits:
- Maximizes Graft Efficiency – Extends autografts, reduces donor site morbidity, and enhances allograft integration
- Ensures Stability – Interlocking granules prevent displacement; porous structure supports blood infiltration
- Accelerates Healing – Promotes osteoconduction, vascularization, and synchronized resorption
- Reduces Surgical Trauma – Minimizes the need for autologous bone harvesting.
- Safe & Biocompatible – 99% phase-pure β-TCP, fully resorbable, and pathogen-free
Clinical Applications:
- Orthopedics & Trauma: Bone defects, post-traumatic reconstruction
- Revision Surgery: Nonunion healing, joint replacement support
CERASORB® M optimizes graft utilization while ensuring safe, effective bone regeneration.
Peri-Implantitis Management: Regenerative Treatment with CERASORB® M
Peri-implantitis is a major challenge in implantology, characterized by progressive bone loss and inflammation around dental implants. A clinical case study by Duarte et al. (2020) evaluated the effectiveness of CERASORB® M in regenerative peri-implantitis treatment, highlighting its role in bone regeneration and infection control.
Case Overview
A 61-year-old female patient presented with pain around an implant in region 46, placed five years prior. Radiographic examination revealed 35% bone loss along the implant length, with a probing depth >5mm.
Treatment Protocol
- Decontamination: The affected area was thoroughly cleaned, and the crown was removed to facilitate healing
- Regeneration: CERASORB® M granules were hydrated with a piperacillin/tazobactam solution and mixed with liquid-phase platelet-rich fibrin (PRF) to enhance stability and sustained antibiotic release
- Healing & Follow-Up: A healing device was placed to protect the implant connection zone
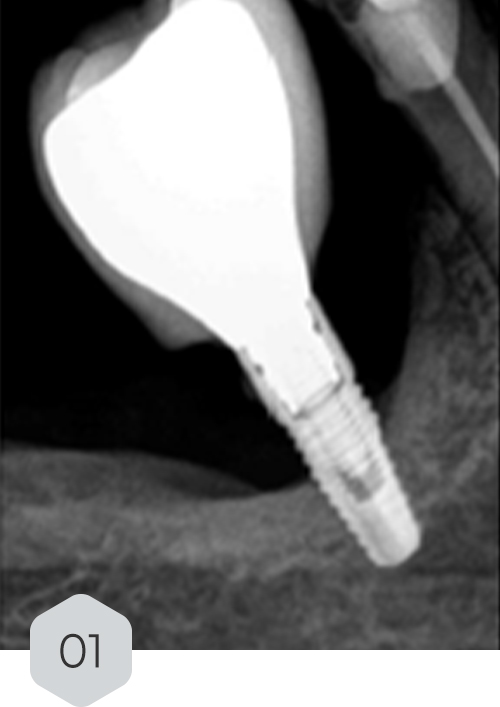
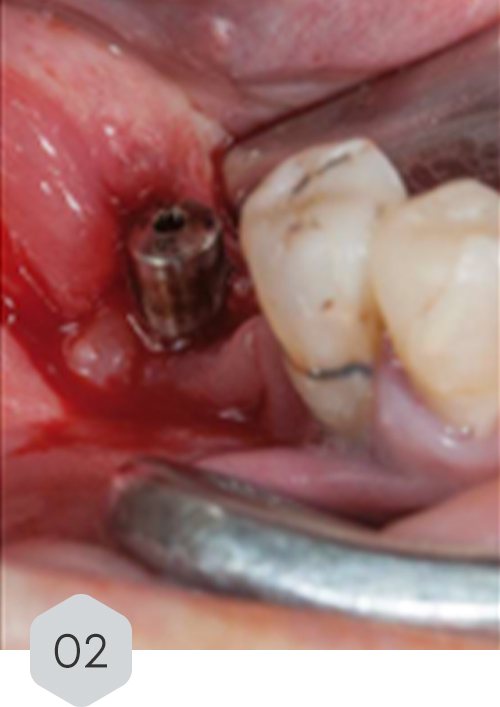
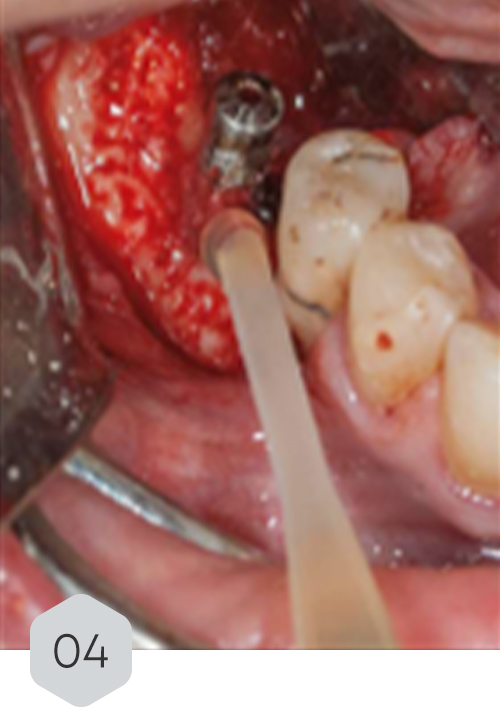
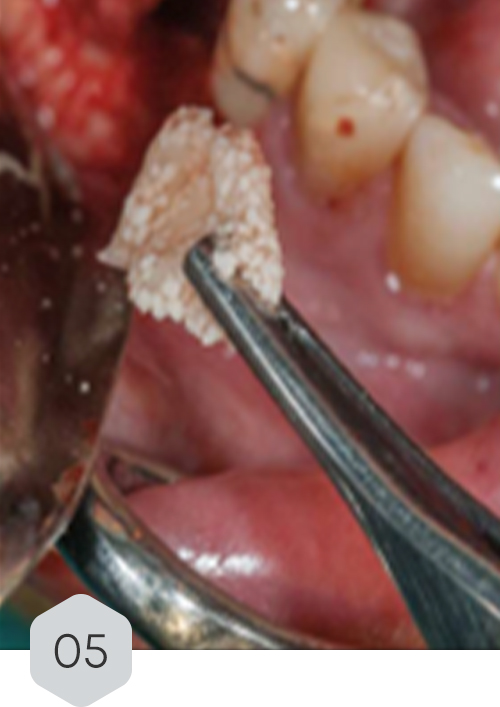
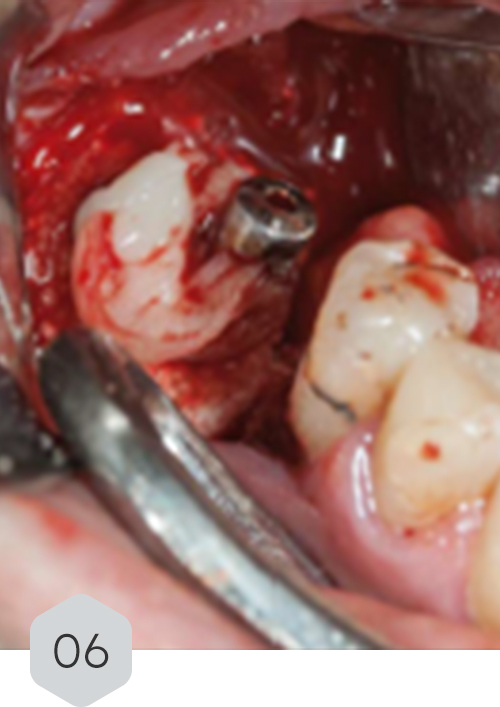
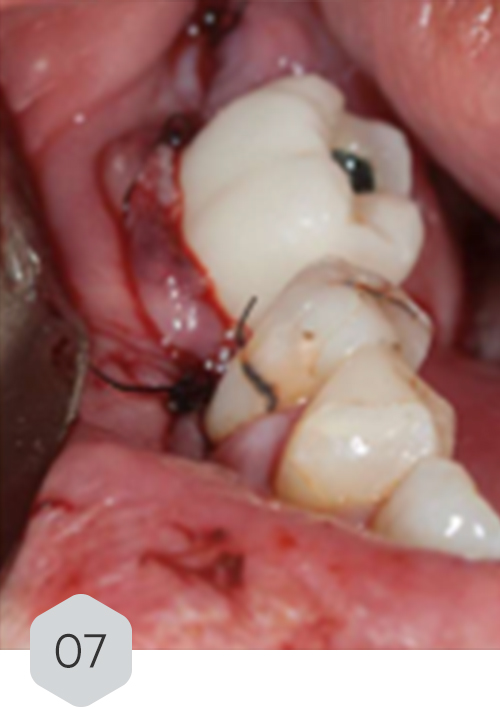
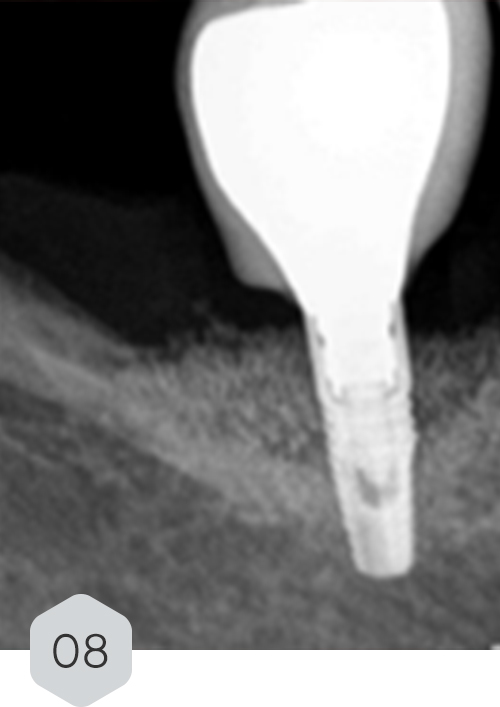
01 & 02 Bone loss on initial radiography. removal of the crown and installation of a healing device to protect the prosthetic connection zone of the implant
03 piezo surgery decorticalization reaching the medullary bone
04 & 05 monomeric phase fibrin dripping to generate a means of continuity and adhesion with the sticky bone
06 polymeric fibrin membranes covering the graft material
07 temporary crown
08 radiograph control with 12-month follow-up
Results & Conclusion
- At 12 months follow-up, the patient showed a favorable clinical outcome with significant bone trabeculation regeneration and was symptom-free
- The study reinforces CERASORB® M’s efficacy in peri-implant bone regeneration, providing structural stability, infection control, and long-term success in peri-implantitis treatment
For more details, access the full study: DOI: 10.20944/preprints202012.0597.v1
CERASORB®: An Effective Bone Graft Substitute for Orthopedic Tumor Surgery
Bone graft substitutes play a critical role in orthopedic oncology, particularly in the treatment of benign and low-grade malignant bone tumors. A recent study by Wittig et al. (2023) evaluated CERASORB®, a β-tricalcium phosphate (β-TCP)-based artificial bone graft, for its effectiveness in bone healing, resorption profile, and postoperative remodeling.
Study Overview
In a retrospective analysis, 43 patients with benign and low-grade malignant bone tumors underwent curettage and CERASORB® implantation between 2018 and 2021. Follow-up assessments were performed using X-rays at intervals of 6 weeks, 3 months, 6 months, and 1 year.
Radiological Case Study in Enchondroma Treatment
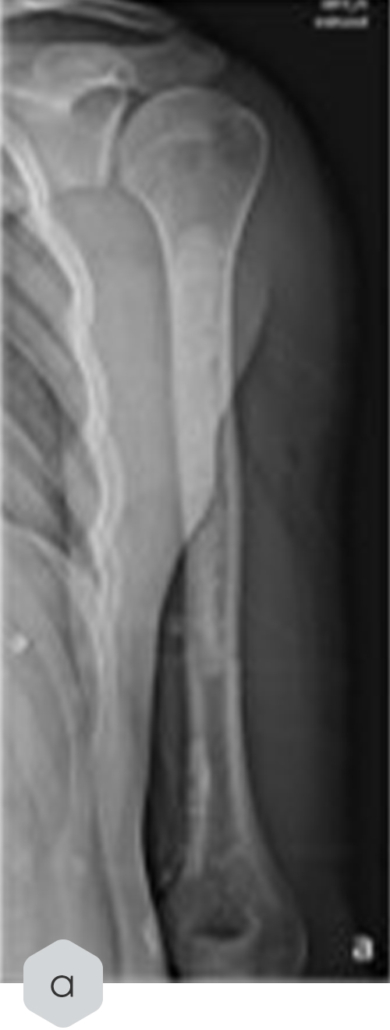
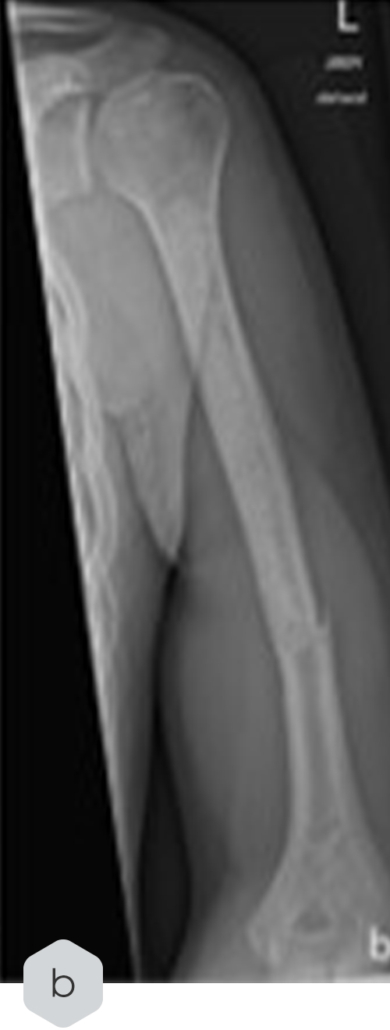
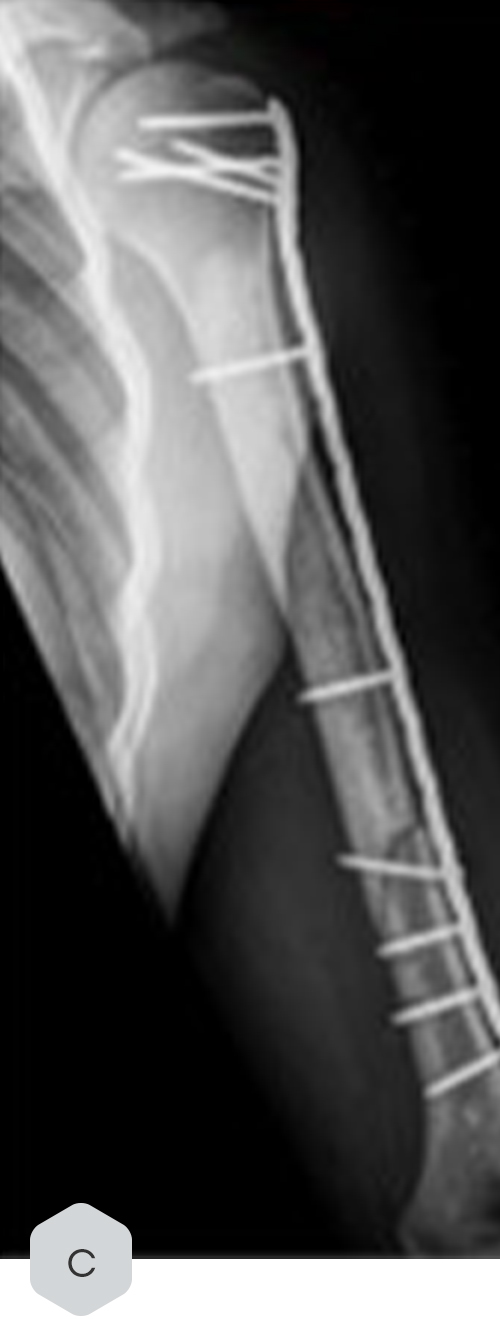
Key Findings
- Bone consolidation was achieved in all patients after an average follow-up of 14.6 months
- Complete resorption of CERASORB® was observed in 16.3% of cases, while partial resorption was noted in 83.7%
- A radiological case study in enchondroma treatment highlighted its integration but also documented a fracture incident post-treatment, which was later stabilized via osteosynthesis
Conclusion
CERASORB® proved to be a safe and effective bone graft substitute, demonstrating low complication rates and serving as a strong alternative to Autografts and Allografts. While some delayed resorption was observed, its overall performance reinforces its value in orthopedic applications.
For more details, access the full study: DOI: 10.1007/s43465-023-00919-1
Why CERASORB® M is the Ideal Choice for Both Fields:
- 100% Biosynthetic & Safe – No risk of infection, rejection, or immune response
- Complete Resorption – Unlike Xenografts, it completely turns into autologous bone
- Different Granule Sizes – Suitable for both dental and orthopedic needs
- Clinically Proven Success – Backed by >30 years of research and >10,000 clinical trials on β-TCP in general
Conclusion: The Future of Bone Regeneration with CERASORB® M
As the demand for biocompatible, safe, and effective bone grafts continues to grow, CERASORB® M sets the standard in both dental and orthopedic bone regeneration. Its ability to fully resorb and transform into natural bone makes it a superior alternative to non-resorbable Xenografts and Allografts.
Whether in dental implantology or orthopedic trauma surgery, CERASORB® M ensures long-term success, patient safety, and optimal healing outcomes.
[i] Miron, R. J., Fujioka‐Kobayashi, M., Pikos, M. A., Nakamura, T., Imafuji, T., Zhang, Y., … & Shirakata, Y. (2024). The development of non‐resorbable bone allografts: Biological background and clinical perspectives. Periodontology 2000, 94(1), 161-179.
[ii] Zhao, R., Yang, R., Cooper, P. R., Khurshid, Z., Shavandi, A., & Ratnayake, J. (2021). Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules (Basel, Switzerland), 26(10), 3007. https://doi.org/10.3390/molecules26103007
[iii] Blume, O., Donkiewicz, P., Palkovics, D., Götz, W., & Windisch, P. (2021). Volumetric Changes of a Customized Allogeneic Bone Block Measured by Two Image Matching Tools: Introduction of a Novel Assessment Technique for Graft Resorption. Acta stomatologica Croatica, 55(4), 406–417. https://doi.org/10.15644/asc55/4/8
[iv] Peng, W., Kim, I. K., Cho, H. Y., Pae, S. P., Jung, B. S., Cho, H. W., & Seo, J. H. (2013). Assessment of the autogenous bone graft for sinus elevation. Journal of the Korean Association of Oral and Maxillofacial Surgeons, 39(6), 274.
[v] Oryan, A., Alidadi, S., Moshiri, A. et al. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9, 18 (2014). https://doi.org/10.1186/1749-799X-9-18
[vi] Graham, S. M., Leonidou, A., Aslam-Pervez, N., Hamza, A., Panteliadis, P., Heliotis, M., … & Tsiridis, E. (2010). Biological therapy of bone defects: the immunology of bone allo-transplantation. Expert opinion on biological therapy, 10(6), 885-901.
[vii] Ciszyński, M., Dominiak, S., Dominiak, M., Gedrange, T., & Hadzik, J. (2023). Allogenic Bone Graft in Dentistry: A Review of Current Trends and Developments. International journal of molecular sciences, 24(23), 16598. https://doi.org/10.3390/ijms242316598
[viii] Amini, Z., & Lari, R. (2021). A systematic review of decellularized allograft and xenograft–derived scaffolds in bone tissue regeneration. Tissue and Cell, 69, 101494.
[ix] Gera, I., Döri, F., Keglevich, T., Anton, S., Szilágyi, E., & Windisch, P. (2002). Experience with the clinical use of beta-tri-calcium phosphate (Cerasorb) as a bone replacement graft material in human periodontal osseous defects. Fogorvosi szemle, 95(4), 143-147.
[x] Horch, H. H., Sader, R., Pautke, C., Neff, A., Deppe, H., & Kolk, A. (2006). Synthetic, pure-phase beta-tricalcium phosphate ceramic granules (Cerasorb®) for bone regeneration in the reconstructive surgery of the jaws. International journal of oral and maxillofacial surgery, 35(8), 708-713.
[xi] Jensen, S. S., Broggini, N., Hjørting‐Hansen, E., Schenk, R., & Buser, D. (2006). Bone healing and graft resorption of autograft, anorganic bovine bone and β‐tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clinical oral implants research, 17(3), 237-243.
[xii] Wüster, J., Neckel, N., Sterzik, F., Xiang-Tischhauser, L., Barnewitz, D., Genzel, A., Koerdt, S., Rendenbach, C., Müller-Mai, C., Heiland, M., Nahles, S., & Knabe, C. (2024). Effect of a synthetic hydroxyapatite-based bone grafting material compared to established bone substitute materials on regeneration of critical-size bone defects in the ovine scapula. Regenerative biomaterials, 11, rbae041. https://doi.org/10.1093/rb/rbae041
[xiii] Bohner, M., Santoni, B. L. G., & Döbelin, N. (2020). β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta biomaterialia, 113, 23–41. https://doi.org/10.1016/j.actbio.2020.06.022
